
Founded in 2015, Curical embarked on a mission to explore the antimicrobial potential of copper as a superior antimicrobial additive for sutures. Over time, Curical strengthened its in-house expertise through collaborations with key consultants and is proud to have former distinguished researchers and executives from Ethicon leading its Research and Development efforts

Founder & CEO

Former VP R&D of Ethicon

Former Distinguished Research Fellow of Ethicon

Former R&D Manger for New Biomaterials at J&J
Copper is not only a natural antimicrobial agent but also an essential element in the human body that is critical for many physiological processes, including wound healing1. It has a broad efficacy, against not only bacteria but also against viruses and fungi – offering the potential for a broader efficacy and claim set than additives that are limited to an “antibacterial” claim due to their limited efficacy against other organisms.
Also, copper has been long used with no known evidence of the emergence of resistant microbial strains2.
High purity copper compounds (metallic, salts, oxides) are readily available in differentiated physical formats offering an array of design possibilities.
Unlike some other materials, the level of copper present within the suture structure does not change during EtO sterilization or storage and is not influenced by the composition of the packaging components.
It is widely recognized that Antibacterial Sutures have emerged as the new gold standard in various global regions, and their use has prevented many Surgical Site Infections (SSI). Over the past 20 years, numerous clinical studies have demonstrated the value of preventing bacterial colonization of the suture surface by incorporation of an antimicrobial agent – resulting in decreased clinical occurrence of SSI. Such studies have corroborated the conclusions from the bench-top microbiological and in-vivo animal model study results, originally generated for these products during their development engendering confidence in their predictive value.
The currently markets Triclosan-based antibacterial suture technology has remained relatively stagnant on the market for over two decades, lacking notable innovations to further enhance its performance. As such, this important and high growth wound closure market segment is ripe for innovation. The introduction of superior copper based antimicrobial/antibacterial products offers an excellent opportunity to benefit patient outcomes and to improve the competitive position and segment penetration of suture manufacturers.
Due to its complex involvement in numerous biochemical cascades, researchers have tended to overlook copper as an in-vivo antibacterial agent despite its well-established benefits and efficacy in other applications. The copper ion interactions with proteins and other moieties can render liberated copper ions ineffective at establishing a sufficient concentration to prevent bacterial colonization. Historically this has resulted in a design challenge to achieve in-vivo efficacy with such approaches.
Through dedicated research efforts and innovative approaches, Curical has pioneered a copper-based antibacterial technology that can be seamlessly integrated into braided absorbable sutures via conventional suture manufacturing processes in a way that, upon implantation, establishes an appropriate copper ion liberation profile and local copper concentration to prevent surface colonization of braided sutures.
Curical’s research has been focused on incorporation of copper containing entities into absorbable multifilament sutures. As these sutures are the most widely used in surgery this approach offers the maximum possibility to benefit patient outcomes through reduced SSI rates.
To realize this vision, we collaborated with experienced suture manufacturers from around the world to develop our prototypes. Additionally, we partnered with leading US Contract Research Organizations (CROs) to conduct our comprehensive in-vitro and in-vivo efficacy, and to conduct comprehensive safety profile studies.
Our robust efficacy data has demonstrated that Curical’s copper-based sutures demonstrate superiority over triclosan-based sutures in a well-established quantitative in-vitro model against those bacteria most often associated with SSI.
The Quantitative data for gram positive Staph Aureus is shown below:
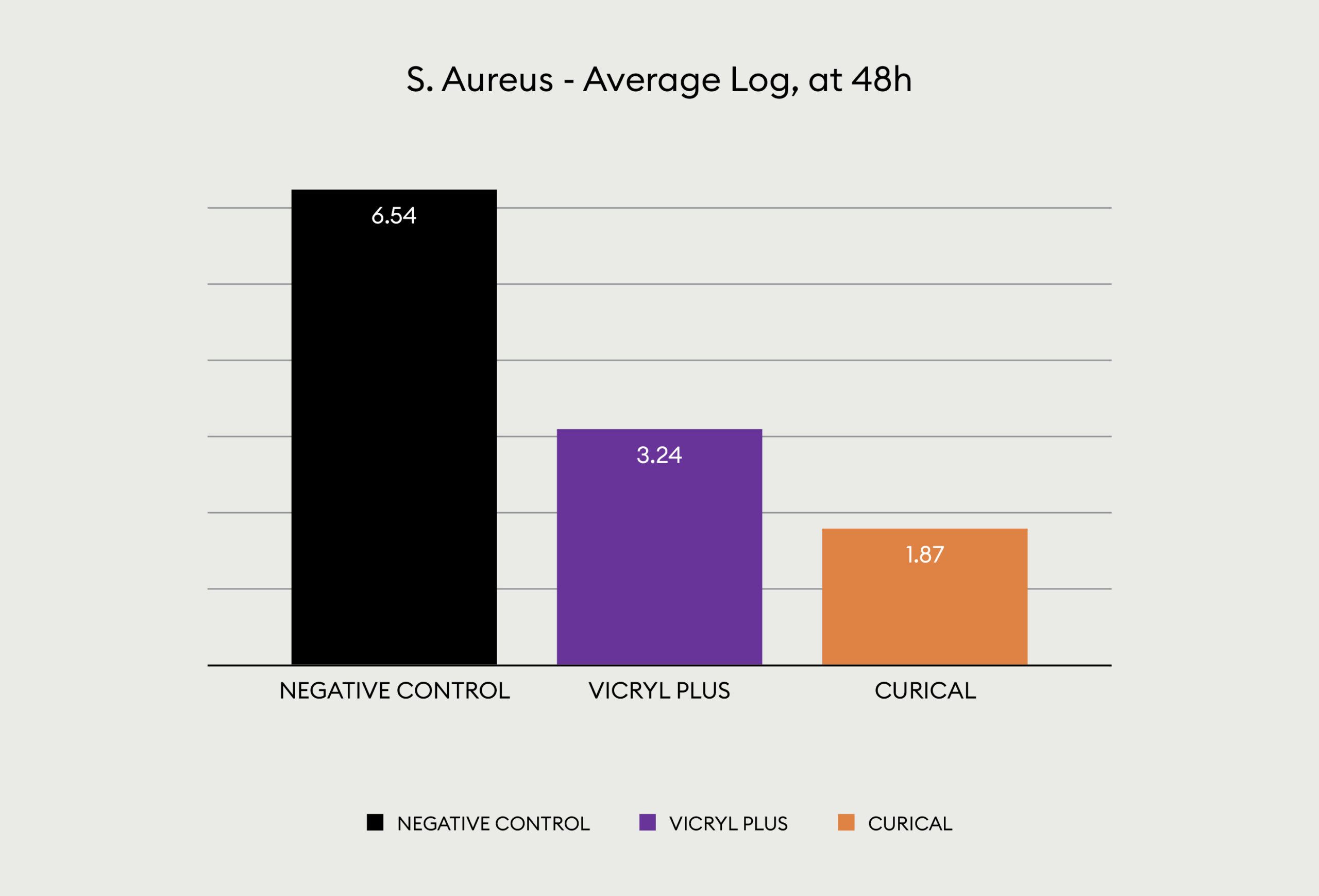
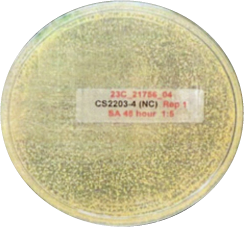
This is another look at the performance, showing visually via the inoculated plates the performance of Curical’s prototype vs. Vicryl Plus (spots represent bacterial colonies):
Negative Control
Vicryl Plus
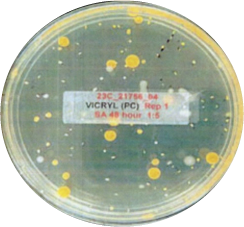
3.57 Log Reduction
Curical’s Prototype
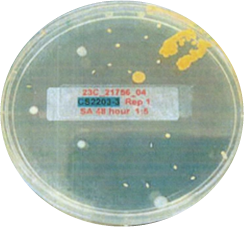
4.67 Log Reduction
Even more impressive is the performance of Curical’s prototype vs Vicryl plus when testing against gram negative E-coli:
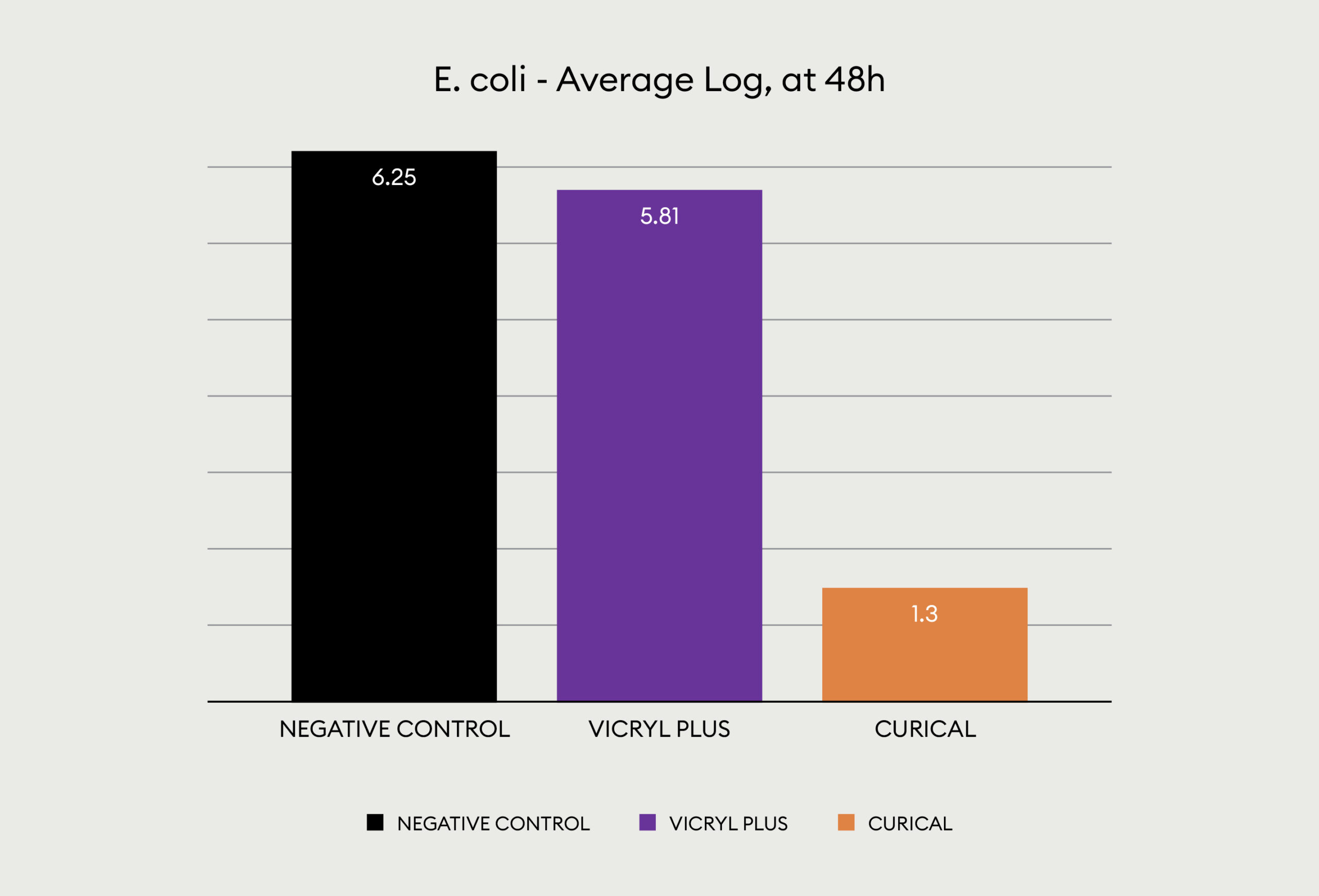
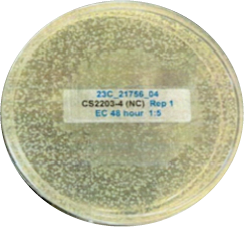
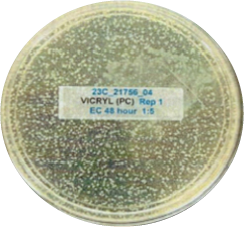
The other look at the performance, showing visually via the inoculated plates the performance of Curical’s prototype vs. Vicryl Plus (spots represent bacterial colonies):
Negative Control
Vicryl Plus
1 Log Reduction
Curical’s Prototype

5.5 Log Reduction
The in-vivo efficacy of Curical’s approach has been demonstrated in an established in-vivo animal model, using Vicryl Plus as the control – and confirming that the performance observed in benchtop microbiology studies translates into the in-vivo environment.
Also – Curical has conducted extensive studies to establish a solid foundation of the safety of its prototypes. All testing conducted in independent labs and could be shared upon request:
Cytotoxicity
Guinea pig maximization sensitization
Intracutaneous reactivity
Rabbit pyrogenicity, material-mediated
Acute systemic toxicity
Genotoxicity
We have a wealth of information to share.
Please get in touch with us – we’d love to hear from you!
info@curicaltech.com
